Table of Contents
Aromatic acid
When carboxylic acid replaced one hydrogen atom in benzene ring then it forms aromatic acids. The aromatic acid group is called a phenyl group.
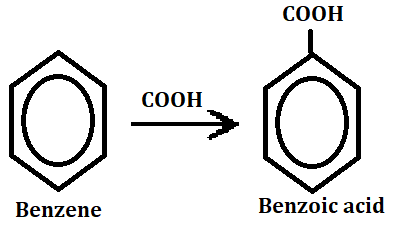
Aromatic acid can be found in many different foods, such as coffee, chocolate and red wine. They are also used in the production of perfumes and other fragrances
Note:- Phenylacetic acid and other similar compounds in which the carboxylic acid group is not directly attached to the aromatic ring called side chain aromatic acid.
Acidity of aromatic acid
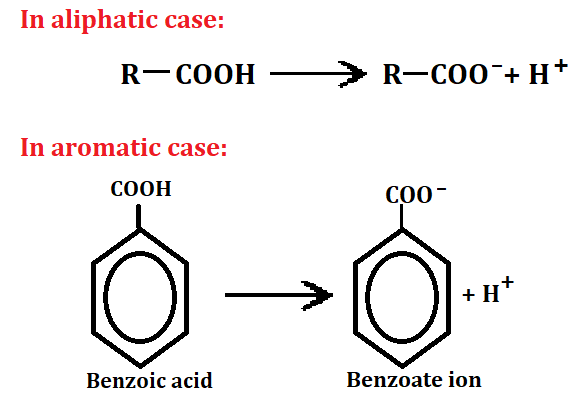
First of all, we understand acid, so those substance which release H+ ion on dissociation is called acid.
As we know that the compound which has more resonance has more stable and more stable then more acidic. Here stability of directly proportional of acidity. Now, benzoate ion has one negative charge and it want to become stable but this negative charge did not delocalize into benzene ring because O- is attached with carbon atom.
So, it delocalized (-) charge into both oxide ion through sharing then benzoate ion become stable and benzoic acid also release H+ ion. It proofs that, aromatic acid is acidic in nature and it is also stable even after dissociation. So, it is strong acid in nature and benzene ring has resonance so it is more stable and acidic.
Important reaction of benzoic acid
In which we formed other compounds with the help of benzoic acid:-
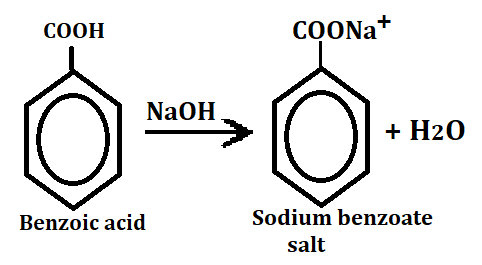
1. Sodium benzoate salt formation
When benzoic acid reacts with any base then it forms salts, react with NaOH.
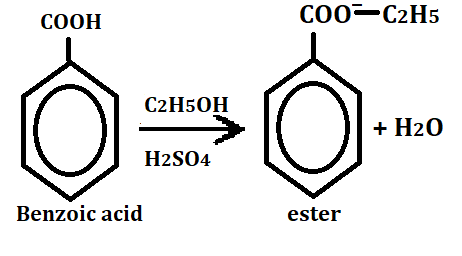
2. Ester formation
It reacts with alcohol in the presence of concentrated sulfuric acid then it forms ester.
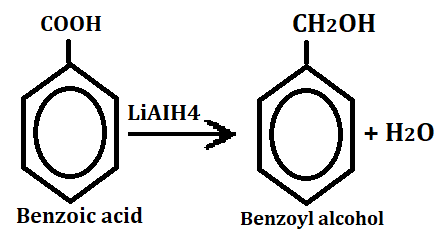
3. Reduction to benzyl alcohol
When it reacts with aluminum hydride and undergoes reductions then it gives benzyl alcohol.
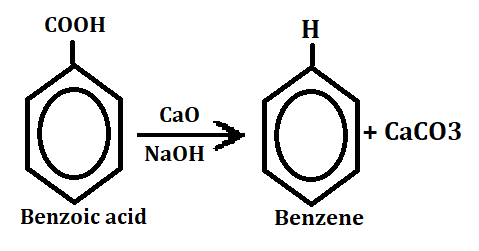
4. Decarboxylation
When benzoic acid reacts with calcium oxide in the presence of NaOH then after decarboxylation it gives benzene and release CaCO3.
Physical properties of aromatic acid
1. Benzoic acid is colorless solid.
2. It has a melting point of 122°C.
3. It is soluble in water, diethyl ether, ethanol.
4. Benzoic acid is stronger acid then acetic acid.
Uses of benzoic acid of aromatic acid
1. It is used as germicides in medicine for urinary infection and in vapor form for disinfectant prochi tube.
2. As food preservatives sodium benzoate is used for preserving pickles, tomato and pharmaceuticals.
3. It is used in production of perfumes, flavones and pharmaceuticals.
4. Aromatic acid is found in the form of salts called benzoates.


To the mimprovement.com owner, Your posts are always informative.
Hi mimprovement.com admin, Thanks for the in-depth post!
Hello mimprovement.com owner, Great post!