Table of Contents
Aromatic amines
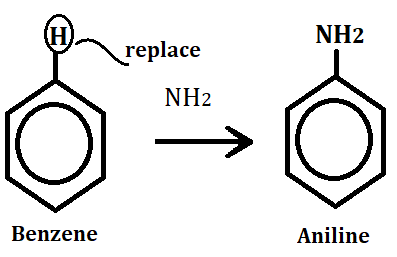
These are those organic compounds in which anime group (-NH2) replace one hydrogen atom(H) on benzene and formed aromatic amines.
- Aromatic amines are a class of organic compounds that have a distinctive odor. Aromatic amines are volatile, colorless liquid with an unpleasant odor. They are used as solvent and in the manufacture of other chemicals, such as pesticides and herbicides.
Basicity of amines
According to the Lewis concept those elements which accept lone pair of electrons is called acid and those which donate lone pair of electrons is called base. So, ammonia contain lone pair of electrons so it is basic in nature and all the of their derivatives are also basic in nature.
Which will be more basic in nature aliphatic amines and aromatic amines
Aliphatic amines are more basic than aromatic amines become aromatic amines there is delocalization of lone pair of electrons due to which benzene ring. Show resonating structure and become less electron availability in the nitrogen atom. Hence cannot accept hydrogen(H+) ion.

Where as in aliphatic amines due to inductive effect nitrogen accepts H+ ion and becomes more basic as compared to the aromatic amines.
Physical properties of amines
1. It is colorless liquid or solid having a characteristic odor, which is not pleasant.
2. They turn brown in air due to oxidation.
3. They are sparingly soluble in water but dissolve in benzene and other organic solvents.
4. They are polar compounds and can form intermediate hydrogen bonding.
5. They are highly toxic substance.
6. They are not readily water soluble due to presence of bulkier phenyl group.
Pharmaceutical application of aromatic amines (anilines)
- Aniline has wide range of pharmaceutical product.
- It is use in precaution of the formation of polyurethane.
- It is also used in making in rubber.
- Some pharmaceutical uses area it is very intermediate for sulpha drugs.
- It is precaution in the synthesis of synthesis of penicillin.
- It is also used in the synthesis of paracetamol.
- It is good in anti-bacterial drug.
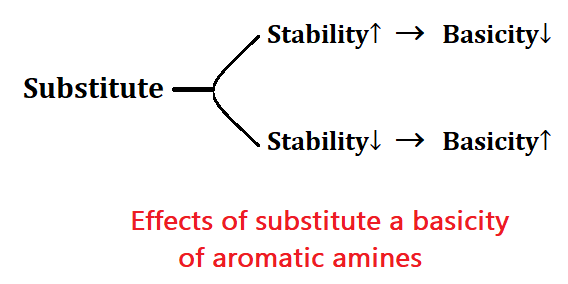
Effects of substitute a basicity of aromatic amines:
In aromatic amines, benzene has resonance so it is less basic but it is more stable. If the stability of a compound is high then its acidity will also be high and if the acidity of a compound is high then its basicity will be less.
According to nature
Substitute also depend upon nature of substituents. There are two natures of substituent: 1. Electron donating group 2. Electron withdrawing group.
1. Electron donating group:- If this group attached with aromatic amines as a substituent it increases the electron density with further increases the basicity, e.g., CH3, C2H5..etc.
2. Electron withdrawing group:- If this group attached with aromatic amines as a substituent it decreases the electron density with further decrease the basicity, e.g., Cl, Br, F…etc.
According to resonance
Resonance increases the stability of benzene ring. So, the more resonance than the more stability. If any group which increasing resonance then increase stability due to this decrease’s basicity. If any group which decreases resonance then decreases stability due to this increase basicity.
Synthetic uses of aryl diazonium salt
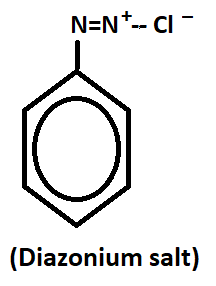
Those compounds which contain N=N in aromatic ring with chloride salt, it is called as aryl diazonium salts.
Use of diazonium salt
1. Diazonium compound are standard reagent used in synthesis of organic compound, especially aryl derivatives.
2. Diazonium salts are light sensitive and breakdown under UV or violet light. so due to this it is used in document reproduction in this, paper or film is coated with diazonium salt.
3. They play a major role to produce dye fabrics.
Method of preparation of aromatic amines
There are some methods for preparations of aromatic amines
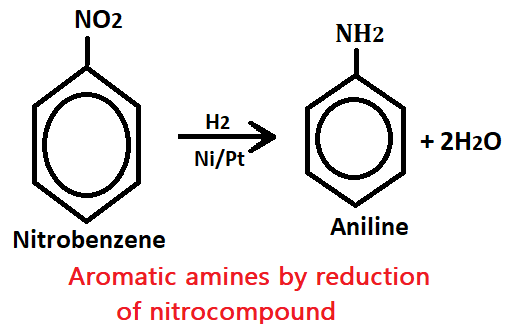
1. By reduction of nitro-compound
When nitrobenzene is reacted with H2 gas in the presence of catalyst Ni/PT it gives aniline (aromatic amine).
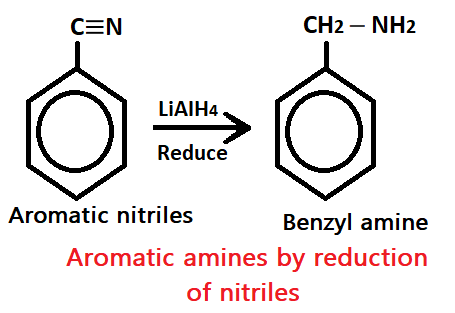
2. By reduction of nitriles
When aromatic nitriles undergo reduction reaction with reducing agent like LiAIH4, it produces aromatic amines (benzyl amines).
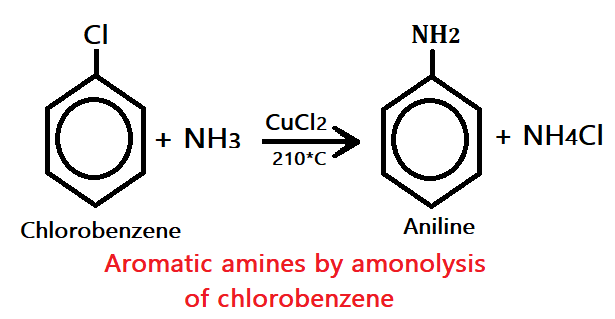
3. By amino lysis of chlorobenzene
Where chlorobenzene undergoes analysis (react with ammonia) in the presence of appear dichloride(CuCl4) at high temperature, it produced aromatic amines(aniline).
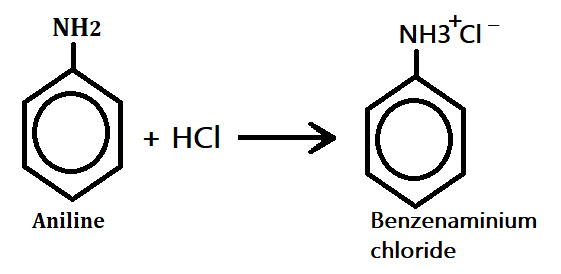
Chemical reaction of aromatic amine
There are some important chemical reactions of aromatic amines
1. Formation of salts:- when aromatic amine (aniline) treated with HCl, it produces benzenaminium chloride(amine salt).
2. Carbylamine reaction:- when aniline react with chloroform(CHCl3) and potassium hydroxide(KOH), it produces carbylamine’s(isocyanide).

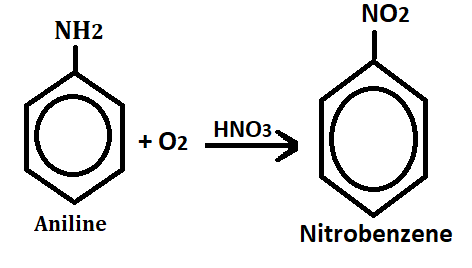
3. Oxidation:- when aromatic amines(aniline) undergo oxidation in the presence nitric acid(HNO3), it produces nitrobenzene.