Table of Contents
Conformational isomerism
These are different spatial arrangement of a molecules that are generated by rotation about single bond. If two different 3D arrangement in space of the atoms in a molecule are inter convertible merely by kee rotation about bonds, they are called conformation.
The difference between conformational isomers & configurational isomers is that configuration isomers can be separated into distinct compound, but conformational isomers are non-separable for change in confirmation only a rotation about a single bond is required, while a change in configuration requires the breaking of a bond & reforming it by a different way.
Alkanes have infinite number of confirmations by rotation around C-C bonds. The rotation around C-C bond is not completely free & it is hindered by a small energy barrier of 1-20 KJ/mole due to weak repulsive interaction between the adjacent bonds, such repulsive interaction is called torsional strain.
Torsional strain is a type of mechanical strain that occurs when an object is twisted. It can cause materials to become weaker and more brittle, which can lead to failure or fracture if too much strain is applied. In engineering and physics, torsional strain is important as it affects how objects respond when subjected to shear forces or rotational motions. It can be measured using torque sensors or torque gauges that measure the amount of force in opposing directions on an object.
Newman projection:- It is basically used to represent 3D structure. Different confirmations of the same molecules are sometimes called conformers & conformational isomers.
Torsional angle:- The angle between the atom attached to the front and rear carbon atoms.
Conformation isomerism on an ethane
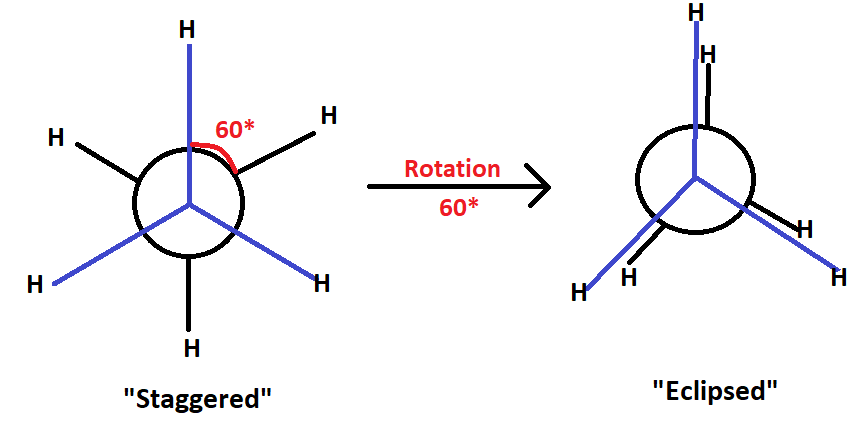
When an ethane molecule rotates about its carbon-carbon single bond, two conformations: staggered and eclipsed. Now, these are also use of energy during rotation. Eclipsed conformation higher in energy then the staggered confirmation.
CH3-CH3 imagine that are methyl group is rotates about the C-C bond as the axis with the other group at rest. Suppose as the starting point in which two C-H bonds are parallel, in this position the dihedral angle (angle of rotation) is zero and if the rotation is free, then the dihedral angle change & the energy content of the molecule will remain constant (the plot between energy content against the dihedral will be horizontal line).
In this situation, the two halves can assume with complex freedom. Out of infinite number of position conformation of ethane, the two extreme arrangement of one methyl group with respect to the other caused by rotation about carbon-carbon bond, they are called to as eclipsed & staggered arrangement. The staggered confirmation is one in which the H-atoms are as far as part as possible. The eclipsed conformation is one in which the H-atom are closed together as possible.
It is clear from the plot between potential energy & angle of rotation that the energy of eclipsed conformation is greater than that of staggered confirmation. The potential barrier is too small like 2.8 kcal/mole for either conformation to remain stable.
The eclipsed & staggered form are reading interconvertible even at normal temperature hence they can’t be isolated as such both the conformation don’t have same stability. The reason behind the more stability of staggered conformation is that the repulsive interaction between the hydrogen atom attach in the two carbon atom are minimum due to the maximum distance between them but in case of eclipsed conformation the repulsive interaction between hydrogen atoms on both carbon atoms are maximum due to minimum distance between them.
The difference between the energy content of the two conformation is known as energy barrier. The energy barrier is very low between both conformation so even at ordinary temperature. The molecules may be interchanged in both conformation, if the dihedral angle 0° or 120° or 240° or 360° than the conformation will be eclipsed form & if the dihedral angle is 60°, 180° and 240° then the conformation will be staggered form.
Due to repulsion between bonds the torsional strain will be more in eclipsed form than that of staggered form, the dihedral angle is denoted by ψ is the interfacial angle between front C-H bond & rear C-H bond & varies from 0°-360°, the energy required to rotate the ethane molecule about C-C bond is called torsional energy.
Conformation isomerism in n-butane
All eclipsed conformation are equivalent in case of ethane & propane what in case of butane, the two distinct conformation are possible. One in which methyl group is eclipsed by a methyl group and second in which the methyl group is eclipsed by hydrogen.

Eclipsed conformation of butane among these two eclipsed confirmation, they are having longer methyl eclipsing together will experience more repulsive interaction forces (Vander wall’s forces) than the other one where a methyl group is faced with a hydrogen atom. Thus, the conformation second is more stable than the conformation first.
Likewise, it is also observed that two different staggered confirmation are also possible for butane. In conformation (3) the methyl group are at the angle of 180° to each other, this confirmation is called anti or trans conformation & in confirmation (4) the methyl group are at the angle of 60° to each other, this confirmation is called gauche’s conformation.
The repulsive interaction forces among these two conformation will be greater in conformation(4) due to close proximity of methyl group, hence the conformation(4) is less stable than confirmation(3).
The confirmation(3) the methyl group are attached together at the maximum distance with the angle of 180°, so this confirmation will be more stable conformation among all the conformation and it will also have potential energy.
Thus, in case of butane, the most stable conformation is generally staggered with the largest group anti to each other & an angle of 60° i.e. anti-conformation & gauche conformation respectively.
Conformational isomerism in cyclohexane
Adolf von Baeyer theory of angle strain
In 1885, the scientist Baeyer proposed a theory to explain a relative stability of few C-alkanes. The theory was based on the classical level & vonitHoff hypothesis is that the 4 valences of a c-atom are directed towards the corners of a regular tetrahedral. Therefore, the normal angle between any pair of bonds of a carbon atom is 109°28` where postulated that any deviation of bond angle from the normal tetrahedral value world impose a condition of internal strain in the ring.
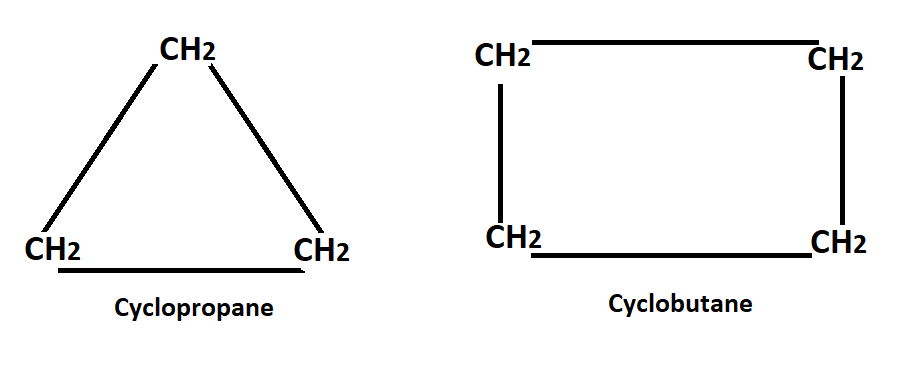
He also assumes that all cyclic ring structure were planar thus calculated the angle through which each of the valency bonds was defected from the normal direction in the formation of various ring, this is called the angle strain & by which the stability of the ring was determined such as cyclopropane.

In C-propane, the 3-carbon atoms occupy the corners of an equilateral triangle, thus C-propane has C-C-C bond angle of 60° (the internal angle of an equilateral angle) this implies that the normal tetrahedral angle of 109°28` between any two bonds in compressed to 60°.
The value of 24°44` represents the angle strain of the deviation through which each bond bends from the normal tetrahedral direction. Here (+) sign indicates that the C-C bond have to be compressed to satisfy the geometry of the ring.
Sache’s Mohr concept of stainless ring
In order to account for the stability of C-alkanes beyond C-pentane, sache’s & Mohr (1918) pointed are that such rings can become absolutely free of strain, if all the ring carbons are not forced into are plane, as was prepared by Baeyer.
If the ring assumed a stainless condition the normal tetrahedral angles of its 109°28′ are retained & as a result. The strain within the ring is relied. The cyclohexane can exist it non-polar stainless form, namely, the boat form & the chair form.

Actually only one form of C-hexane is known & not two forms as so above the failure to isolate the two forms is due to rapid interconversion between them. Such non-polar stainless rings in which the ring carbon atom can have normal tetrahedral angle are possible for larger rings compounds.
According to model molecular orbital theory for a bond to form, two atoms must be involved so that n-orbital of one atoms overlaps with an orbital of the other atom for a given pair of atoms the greater the overlapping of orbital the stronger the bond. When a carbon is bonded to form other atom its bonding orbital (sp3) are directed to the corners of a regular tetrahedral, the angle between any pair of orbitals is thus 109°28`.
Chair form:- staggered conformers (stable more)
Boat form:- eclipsed conformers (less stable)
Chair conformation is more stable than the boat conformation.
Energy diagram & conformers
Confirmations of cyclohexane are as follows:
1. Chair form
There is no steric hindrance, so it has minimum energy and maximum stability. It is staggered conformers (more stable).
2. Half chair form
It has both angle strain and torsional strain, so less stable than chair form. Energy- 46kj/mole
3. Twist boat form
It is more stable than boat conformation by about 5.4kj/mole, but less stable than chair conformation by 23.4 kj/mole.
4. Boat form
There is steric interaction between the non-bonding atom due to this, the boat conformation is less stable than chair conformation and has higher energy content.
Conformational isomers
Conformational isomerism is an important concept in organic chemistry that deals with the arrangement of covalent bonds in a molecule. It is a type of stereoisomerism, which means that compounds have the same molecular formula but different spatial arrangements. This can result in different chemical and physical properties. For instance, conformational isomers can have different boiling points and melting points, as well as different reactivity and solubility. This phenomenon has wide-ranging implications for various chemical processes, such as drug synthesis and chemical reactions.

Hello mimprovement.com admin, Thanks for the well-structured and well-presented post!
Hi mimprovement.com administrator, Your posts are always well-supported by research and data.