Table of Contents
Heterocyclic compounds
Heterocyclic compounds are a group of organic molecules whose atoms form rings containing both carbon and other elements such as nitrogen, oxygen, sulphur, and phosphorus, in which it contains at least two different atoms. They are essential components of many pharmaceuticals as well as natural products. The atom presents in the ring other than that of carbon is known as heteroatom.
The most common hetero atom includes nitrogen, oxygen, sulphur, phosphorus and copper etc. Most of the drug which are used in pharmaceutical science are heterocyclic compound. These compounds may be aromatic and non-aromatic according to their ring type.
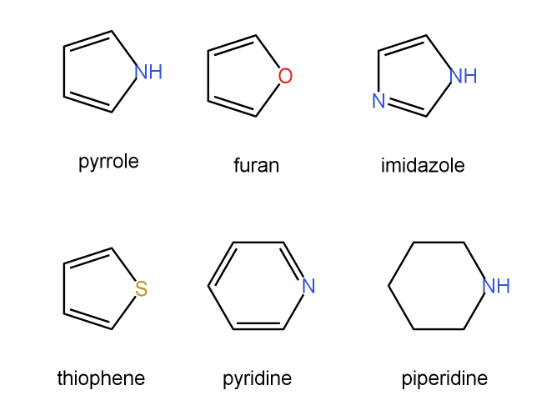
Example: pyrrole, furan, oxazole etc.
Classification of heterocyclic compound
1. Five membered heterocyclic compound
These are five membered ring compounds consisting of one hetero atom and four carbon atoms. Example: furan and pyrrole.

2. Six membered heterocyclic compound
These are six membered ring compounds consisting of one hetero atom and five carbon atoms. Example: pyridine and piperidine

3. Seven membered heterocyclic compound
These are seven membered ring compounds consisting of one hetero atom and six carbon atoms. Example: azepine, thiepane and oxepine etc.
4. Condensed heterocyclic compound
These types of compounds comprise of a benzene ring fused with the hetero cyclic ring containing 5 or 6 atoms. Example: indole and quinoline.
Nomenclature of heterocyclic compound
1. Common nomenclature
It is also known as a trivial name system. Each compound is given the corresponding trivial name. This usually originates from the compound’s occurrence, it’s first preparation or its special properties. A common nomenclature exists to provide a standard way of naming these compounds so that their structure can be accurately described.
2. Replacement nomenclature
Heterocyclic’s name is composed of the corresponding carbocycle’s names and an elemental prefix for the heteroatom introduced. If the more than one hetero atom is present, they should be listed according to the priority order.
| Hetero atom | Valency | Prefix |
| O | 2 | Oxa |
| N | 3 | Aza |
| S | 2 | Thia |
| P | 3 | Phospha |
| As | 3 | Arsa |
| Si | 4 | Sila |

3. Hantzsch-Widman nomenclature (IUPAC)
Hantzsch-Widman nomenclature is a system of chemical nomenclature developed to standardize the naming of organic compounds. It allows chemists to systematically categorize compounds based on their functional groups, enabling them to easily identify and compare similar molecules and predict their behaviour.
German chemist Arthur Hantzsch and Oskar Widman, proposed similar systematic naming of heterocyclic compound in 1887 and 188 respectively. Three to ten membered rings named by combining the appropriate prefix that denotes the type & position of the heteroatom present in the ring with suffix, that determines both other ring size and the degree of unsaturation.
Saturated ring system
Saturated suffix applies only to completely saturated ring system. In a monocyclic compound, the numbering is controlled by the position of a single heteroatom, while this does not hold true in case of bicyclic compounds

Unsaturated ring system
The position of nitrogen or carbon atoms which bear extra hydrogen atoms must be indicated by number & italic capital H(eg – 1H, 2H) followed by the name of maximally unsaturated ring.
Relative aromaticity of heteroatom
The aromatic compounds must pass following properties:
1. Molecule must be cyclic.
2. It is must to each atom present in the ring to have p-orbital that overlaps with the p-orbital of another atom on either side.
3. Molecule must be planar.
4. The compound should contain odd number of pi electron must satisfy the Buckets Huckel’s rule.
Aromaticity of heterocyclic compound
Three members of heterocyclic compounds like furan, pyrrole & thiophene, there is increase in the aromatic character as per the following order:
Furan<pyrrole<thiophene
Heterocyclic compound similar to benzene show aromaticity are stabilised by resonance. The resonance energy of heterocyclic compound is comparatively much lower than benzene. The resonance energy of following compounds decreases in the order given below.
Thiophene>pyrrole>furan
The hybridization of atom present in the ring is in sp2 state & the ring remains plane.


Hi mimprovement.com administrator, Your posts are always a great source of knowledge.