Table of Contents
Introduction of imidazole
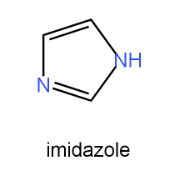
Introduction of imidazole, it is a five membered heterocyclic system with three carbon atoms and two nitrogen atoms at the positions 1 & 3. It is also named as 1,3-diazole. In the pharmaceutical industry, imidazole derivatives have been extensively used as active ingredients in the development of drugs. Their ability to interact with biological targets makes them valuable for treating various diseases such as cancer, infections, and cardiovascular disorders. Imidazole-based drugs have shown promising results in clinical trials and continue to be an area of active research.
Imidazole also plays a significant role in the field of agriculture. It is utilized as a fungicide to control fungal diseases in crops. Its ability to inhibit the growth of fungi makes it an effective tool for protecting agricultural yields and ensuring food security.
Synthesis of imidazole
1. Radziszewski synthesis (from dicarbonyl compounds)
In this synthesis, dicarbonyl compounds are condensed with aldehyde in presence of ammonia.
2. By dehydrogenation of imidazole
In this synthesis, ethylenediamine is react with functional group in the presence of ammonia to form imidazole.

Chemical reactions of imidazole
1. Oxidation
Oxidation of imidazole in presence of H2O2 leads to ring opening.
2. Nitration
Imidazole is reacted with nitric acid and sulphuric acid to form 4-nitro imidazole.

3. Sulphonation
In sulphonation of imidazole, it is treated with oleum at 160°C and formed imidazole-4-sulphonic acid.

4. Chlorination
In this reaction, imidazole is reacted with chlorine in the presence of weak acid and formed 4-chloro imidazole.

Physical properties of imidazole
It is highly polar compound having dipole of 3.61D. It is freely soluble in polar solvents like water. The hydrogen atom can be located on any of the 2 nitrogen atoms which results in its two tautomeric forms.

The aromaticity of the compound is due to the completion of sextet by the π electrons, comprising of electrons pair obtained from protonated nitrogen atom, one from each of other four atoms present in the ring.
Medicinal uses of imidazole
1. Derivatives of imidazole are potent antifungal agents & are also used to treat mycotic infection of skin.
2. Antibiotic of class nitroimidazole are used to combat anaerobic bacterial and parasitic infections.
3. Metronidazole is used to treat bacterial infections of the skin, vagina, stomach, joints or respiratory tract.



Hi mimprovement.com admin, You always provide useful tips and best practices.
To the mimprovement.com administrator, Your posts are always well written and informative.