Stereoisomerism in biphenyl compounds
Biphenyl is an aromatic hydrocarbon with a molecular formula (C6H5)2, consists of two connected phenyl ring. Due to lack of formation of groups, it is unreactive in nature. (The two phenyl groups are linked by a single bond sp2 known as pivotal bond).
Biphenyl does not show geometrical isomerism, its show conformational isomerism due to rotation around the single bond. Biphenyl containing for a large group in the two ortho positions cannot freely rotate. Two ortho position can free rotate about central bond because of strict hindrance plane. In such compounds, the two rings are in perpendicular plane.
The distance between two o-hydrogen in adjacent rings in the planar conformation is significantly in greater than twice the Vander wall’s radius of hydrogen. The distance between two o-hydrogen is approx. 2.9A° and the radius of Vander walls of hydrogen is approx. 2.4A° due to this reason the rotation around the pivotal bond is not hampered by stearic factor.
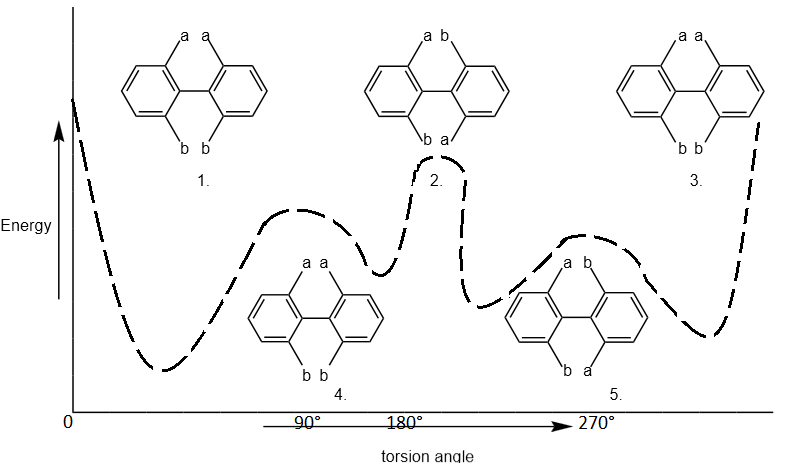
The diagram shows a 360° rotation around the pivotal bond. The inter-ring resonance or the energy of the compound is lowest at 90° angle therefore, when the torsion angle is 90° & 270° as shown in the figure.
The compound will have least energy & will be more stable thus the preferred conformation of the enantiomers is those in which the two phenyl planes are approximately but not exactly perpendicular to each other. Hence, the compound in the form of structure 3 & 4 will be more stable than in the form of structure 1, 2 & 4). In these structure (1, 2 & 5) the inter atomic distance between atoms a-a & b-b and a-b is minimum inter atomic distance.
The strict hindrance or repulsion will be greater than those of between atoms of structure 3 & 4. Thus, the structure (1, 2, 5) will have greater energy than that the structure (3 & 4) & therefore, the structure (1, 2, 5) will be stable than that of structure (3 & 4).
It the energy barrier exceeds 80-100 KJ/mol, the stereoisomers can be repeated at normal room temperature because of their greater energy difference. Such isomerism which results due to restricted rotation around a single bond is termed as atropoisomeric & the isomers are known as atropoisomeric.
The term atropoisomeric comes from a Greek word in which ‘a’ mean ‘not‘ & ‘tropes’ means ‘turn‘. It means those compounds which resist turning around a single bond. The atropoisomeric can be defined as isomers that can be isolated due to prevention or restriction of rotation about a given single bond usually between two planar molecules.
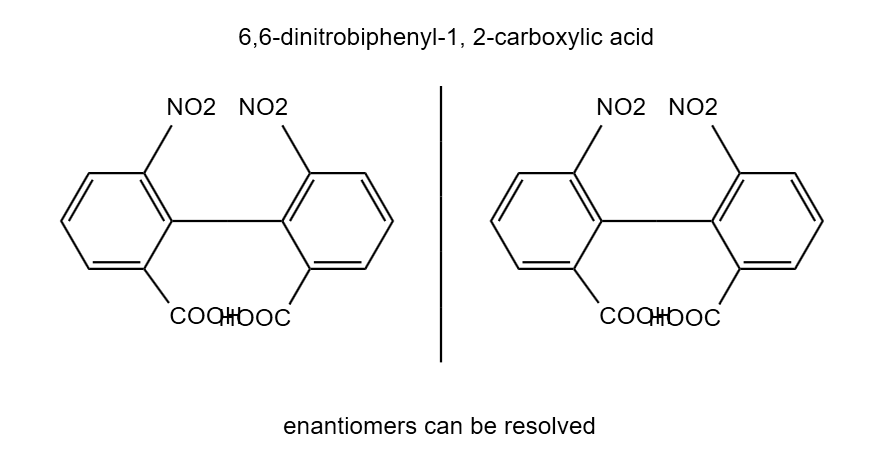
Biphenyl substituted on o-position become inclined to each other in respect of stearic repulsion or hindrance among the atoms attached in the o-position, the pivotal bond is restricted. The interconversion between the two isomer is restricted. Therefore, the two isomers exist as separate entities & can be resolved to separate enantiomers. Stereoisomerism in biphenyl compounds
Condition for biphenyl compounds to exhibit optical activity
1. There should be any functional group/atom at ortho position of rings. (Substitution at ortho position with large size such as – Cl, Br, I, COOH, NO2, SO3H, Ch3).
2. Each ring must be resolvable for that a is not equal b and c is not equal d.
3. This order is corresponds roughly to the order of Vander wall’s radii of the group.
4. The biphenyl compounds containing large substituent or groups can be readily racemized in heating & separate into the form of enantiomer.
Stereoisomerism in biphenyl compounds is a fascinating topic in organic chemistry. It refers to the phenomenon of molecules having the same structural formula but different arrangements of their atoms. This results in the formation of two or more stereoisomers, which have different physical and chemical properties. The study of this phenomenon has important implications for drug design and synthesis processes, as well as other areas of research. By understanding how stereoisomerism works, scientists can work towards creating more efficient and cost-effective drugs that are tailored to specific needs. In this article, we will explore how biphenyl compounds display stereoisomerism and its implications for research. Stereoisomerism in biphenyl compounds and conditions for optical activity.